(1) Phycobilisomes (PBSs) are light-harvesting antennae that transfer energy to photosynthetic reaction centers in cyanobacteria and red algae. PBSs are supermolecular complexes composed of phycobiliproteins (PBPs) that bear chromophores for energy absorption and linker proteins. Although the structures of some individual components have been determined using crystallography, the three-dimensional structure of an entire PBS complex, which is critical for understanding the energy transfer mechanism, remains unknown. Here, we report the structures of an intact PBS and a PBS in complex with photosystem II (PSII) from Anabaena sp. strain PCC 7120 using single-particle electron microscopy in combination with biochemical and molecular analyses. In the PBS structure, all PBP trimers and the conserved linker protein domains were unambiguously located, and the global distribution of all chromophores was determined. We provide evidence that ApcE and ApcF are critical for the formation of a protrusion at the bottom of PBS, which plays an important role in mediating PBS interaction with PSII. Our results provide insights into the molecular architecture of an intact PBS at different assembly levels and provide the basis for understanding how the light energy absorbed by PBS is transferred to PSII.
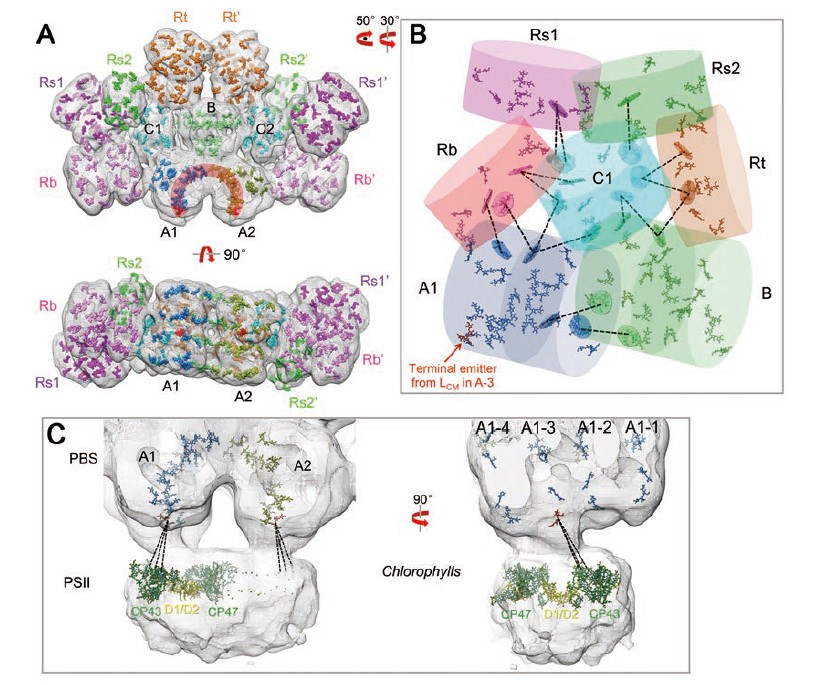
Chang LF, Liu X, Li Y, Liu CC, Yang F, Zhao J, Sui SF. (2012) Structural organization of an intact phycobilisome and its association with photosystem II. Cell Research, 25(6):726-737
(2) The 20S particle, which is composed of the N-ethylmaleimide–sensitive factor (NSF), soluble NSF attachment proteins (SNAPs) and the SNAP receptor (SNARE) complex, has an essential role in intracellular vesicle fusion events. Using single-particle cryo-EM and negative stain EM, we reconstructed four related three-dimensional structures: Chinese hamster NSF hexamer in the ATPγS, ADP-AlFx and ADP states, and the 20S particle. These structures reveal a parallel arrangement between the D1 and D2 domains of the hexameric NSF and characterize the nucleotide-dependent conformational changes in NSF. The structure of the 20S particle shows that it holds the SNARE complex at two interaction interfaces around the C terminus and N-terminal half of the SNARE complex, respectively. These findings provide insight into the molecular mechanism underlying disassembly of the SNARE complex by NSF.
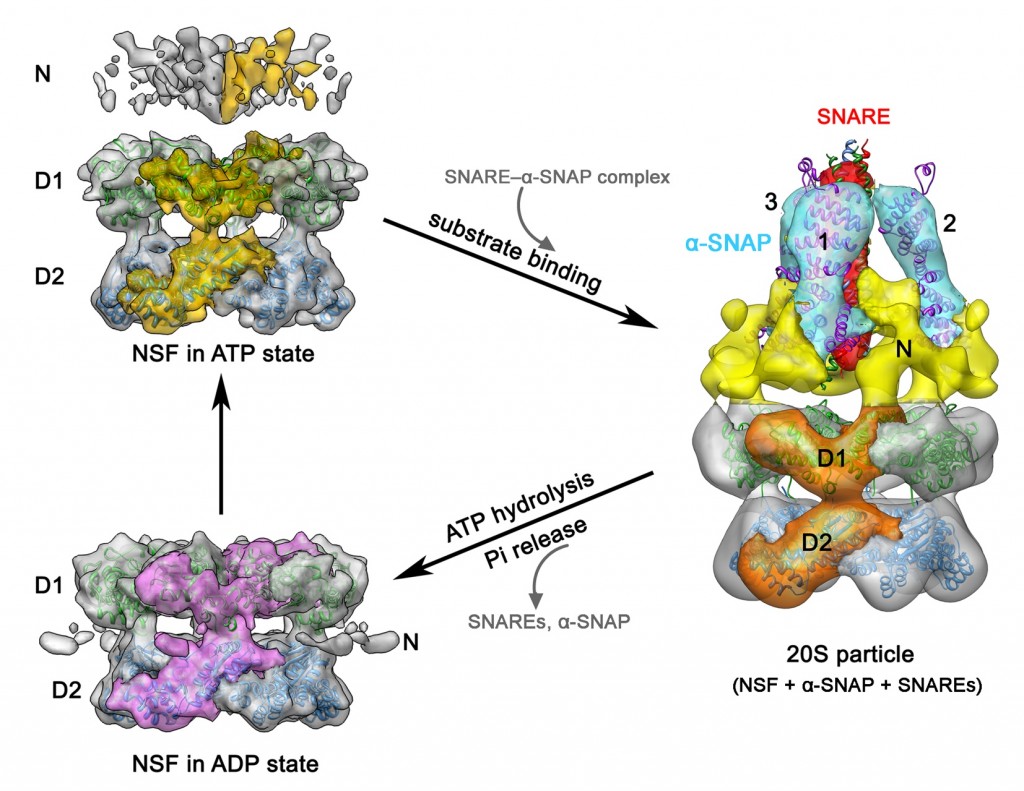
Chang LF, Chen S, Liu CC, Pan X, Jiang J, Bai XC, Xie X, Wang HW and Sui SF. (2012) Structural characterization of full-length NSF and 20S particles. Nature Structural & Molecular Biology, 19: 268-275
(3) HtrA family proteins play a central role in protein quality control in the bacterial periplasmic space. DegQ-like proteases, a group of bacterial HtrA proteins, are characterized by a short LA loop as compared with DegP-like proteases, and are found in many bacterial species. As a representative of the DegQ-like proteases, we report that Escherichia coli DegQ exists in vivo primarily as a trimer (substrate-free) or dodecamer (substrate-containing). Biochemical analysis of DegQ dodecamers revealed that the major copurified protein substrate is OmpA. Importantly, wild-type DegQ exhibited a much lower proteolytic activity, and thus higher chaperone-like activity, than DegP. Furthermore, using cryo-electron microscopy we determined high-resolution structures of DegQ 12- and 24-mers in the presence of substrate, thus revealing the structural mechanism by which DegQ moderates its proteolytic activity.
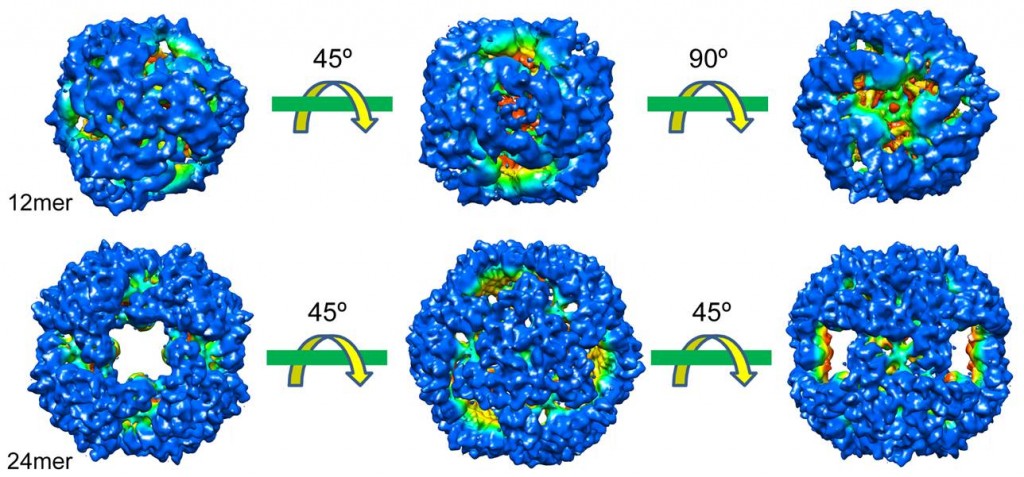
Bai XC, Pan XJ, Wang XJ, Ye YY, Chang LF, Leng Dong, Lei JL and Sui SF (2011) Characterization of the Structure and Function of Escherichia coli DegQ as a Representative of the DegQ-like Proteases of Bacterial HtrA Family Proteins. Structure, 19: 1328–1337
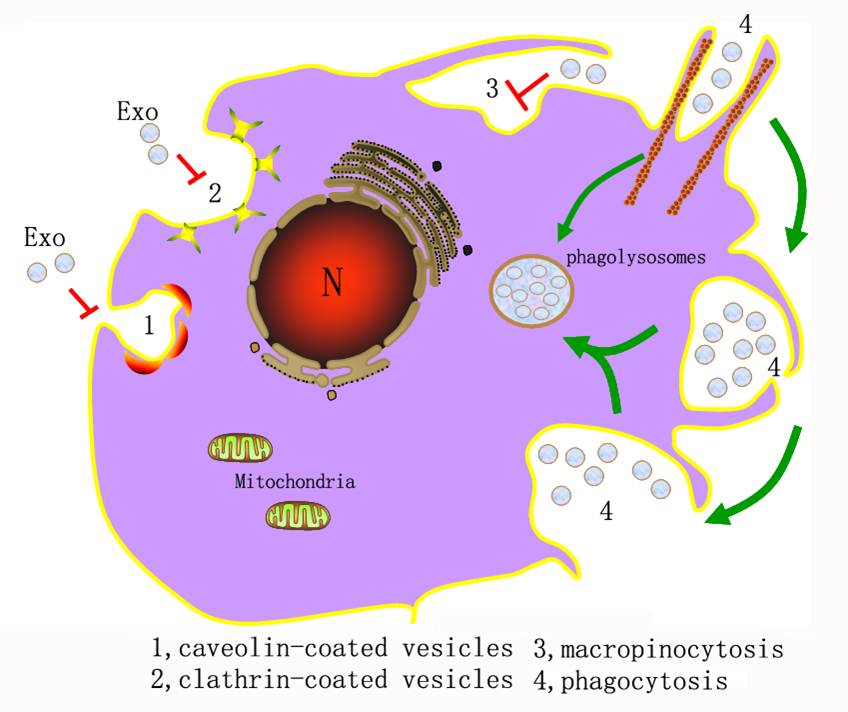
(4) Exosomes play important roles in many physiological and pathological processes. However, the exosome-cell interaction mode and the intracellular trafficking pathway of exosomes in their recipient cells remain unclear. Here, we report that exosomes derived from K562 or MT4 cells are internalized more efficiently by phagocytes than by non-phagocytic cells. Most exosomes were observed attached to the plasma membrane of non-phagocytic cells, while in phagocytic cells these exosomes were found to enter via phagocytosis. Specifically, they moved to phagosomes together with phagocytic polystyrene carboxylate-modified latex beads (biospheres) and were further sorted into phagolysosomes. Moreover, exosome internalization was dependent on the actin cytoskeleton and phosphatidylinositol 3-kinase, and could be inhibited by the knockdown of dynamin2 or overexpression of a dominant-negative form of dynamin2. Further, antibody pretreatment assays demonstrated that tim4 but not tim1 was involved in exosomes uptake. We also found that exosomes did not enter the internalization pathway involving caveolae, macropinocytosis and clathrin-coated vesicles. Our observation that the cellular uptake of exosomes occurs through phagocytosis has important implications for exosome-cell interactions and the exosome intracellular trafficking pathway.
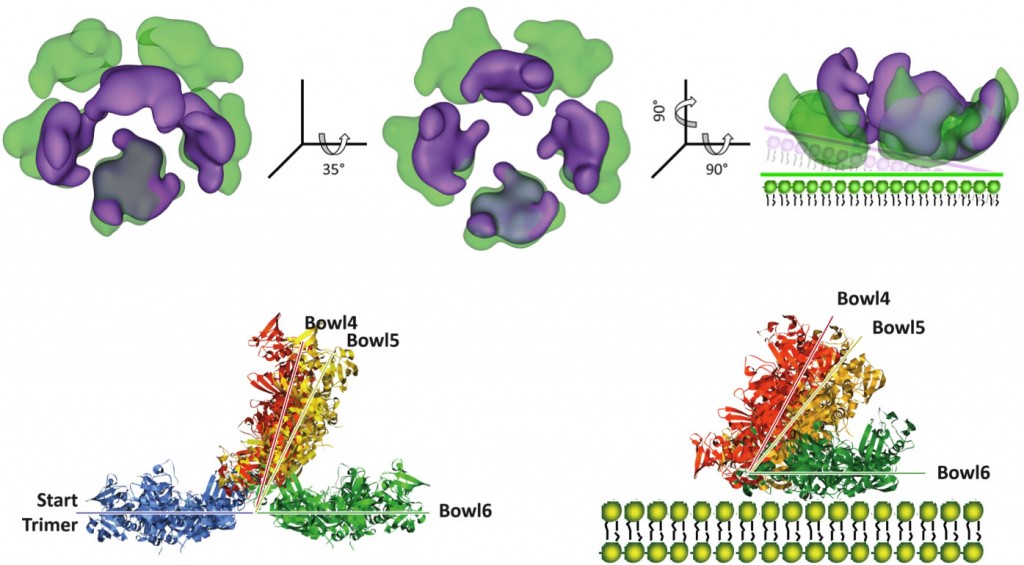
(5) In the periplasm of Escherichia coli, DegP (also known as HtrA), which has both chaperone-like and proteolytic activities, prevents the accumulation of toxic misfolded and unfolded polypeptides. In solution, upon binding to denatured proteins, DegP forms large cage-like structures. Here, we show that DegP forms a range of bowl-shaped structures, independent of substrate proteins, each with a 4-, 5-, or 6-fold symmetry and all with a DegP trimer as the structural unit, on lipid membranes. These membrane-bound DegP assemblies have the capacity to recruit and process substrates in the bowl chamber, and they exhibit higher proteolytic and lower chaperone-like activities than DegP in solution. Our findings imply that DegP might regulate its dual roles during protein quality control, depending on its assembly state in the narrow bacterial envelope.
as DegP’s new functional forms in protein quality control. Proc Natl Acad Sci U S A, 106: 4858-4863
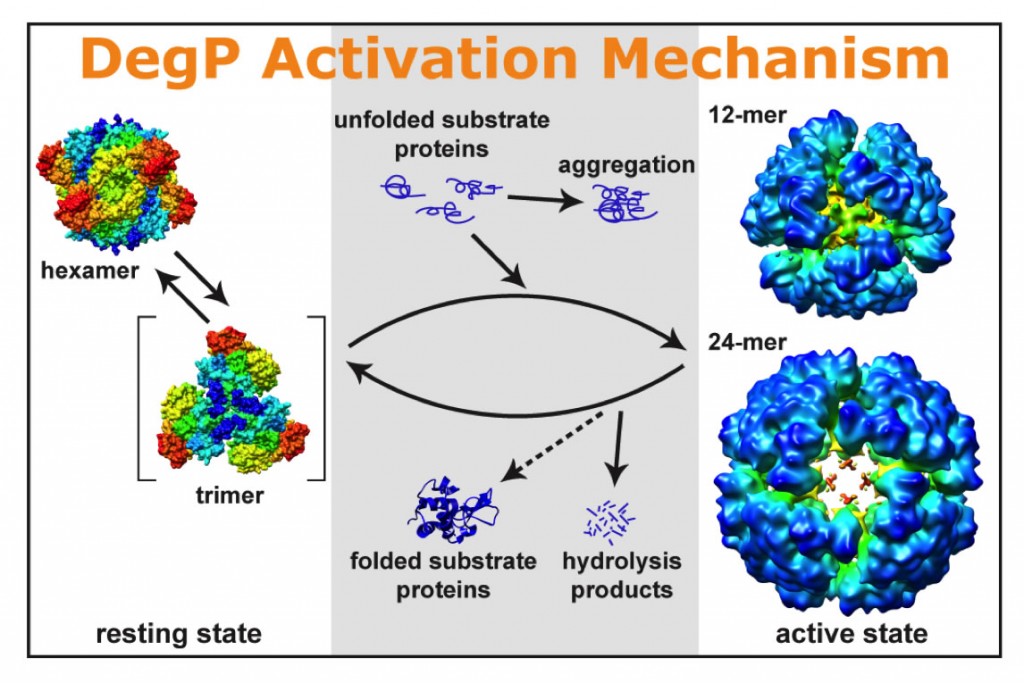
(6) Cells use molecular chaperones and proteases to implement the essential quality control mechanism of proteins. The DegP (HtrA) protein, essential for the survival of Escherichia coli cells at elevated temperatures with homologues found in almost all organisms uniquely has both functions. Here we report a mechanism for DegP to activate both functions via formation of large cage-like 12- and 24-mers after binding to substrate proteins. Cryo-electron microscopic and biochemical studies revealed that both oligomers are consistently assembled by blocks of DegP trimers, via pairwise PDZ1-PDZ2 interactions between neighboring trimers. Such interactions simultaneously eliminate the inhibitory effects of the PDZ2 domain. Additionally, both DegP oligomers were also observed in extracts of E. coli cells, strongly implicating their physiological importance.
(7) Toxins penetrate mammalian cells through various means. In this study, we report a unique strategy used by trichosanthin (TCS), a plant toxin with ribosome-inactivating activity, to penetrate host cells. We found that in both JAR and K562 cells, endocytosed TCS is incorporated into intraluminal vesicles of the multivesicular body (MVB) and is then secreted in association with these vesicles upon fusion of the MVB with the plasma membrane. The secreted TCS-loaded vesicles secreted by K562 cells move throughout the intercellular space and target syngeneic and specific allogeneic cells. Subsequent internalization permits delivery of the toxin into the cytosol, resulting in ribosomal inactivation and cell death. Thus, our findings provide a novel mechanism by which foreign proteins pass between and penetrate into mammalian cells.
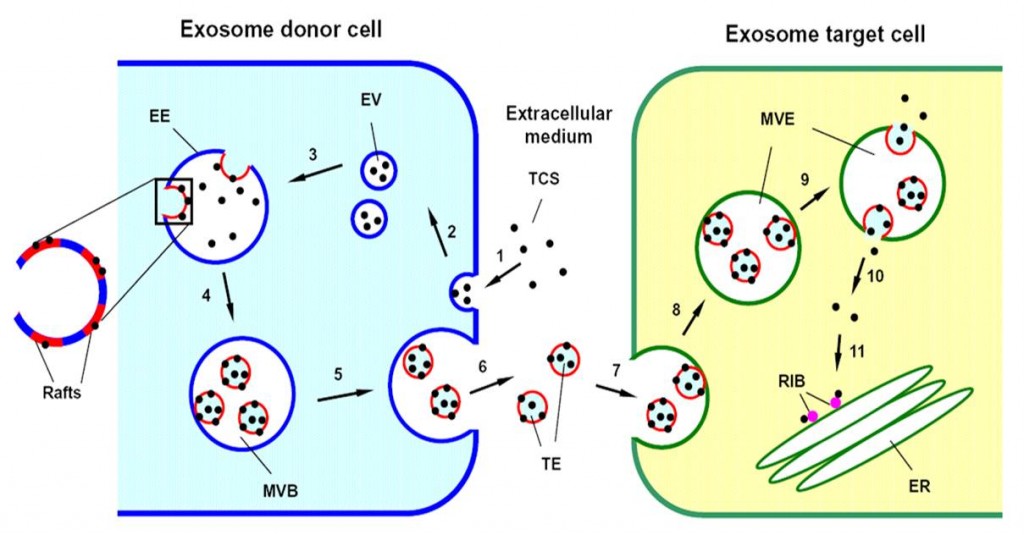
Exosome-Mediated Intercellular Trafficking. Traffic, 10: 411-424
